The Department of Molecular Genetics was established in December 1999 with the aim of unravelling the genetics of diabetes and its complications, cardiovascular diseases, and obesity. The department conducts research in molecular and functional genomics, supported by competitive funding from several agencies, including the ICMR, DBT, and DST.
It has published numerous papers in peer-reviewed journals such as Nature, Nature Genetics, Nature Communications, American Journal of Human Genetics, Cell Genomics, Human Genetics, Clinical Genetics, Metabolism, Journal of Clinical Endocrinology & Metabolism, Diabetes, Diabetologia, Diabetic Medicine, Diabetes Care, Paediatric Diabetes, Metabolism: Clinical and Experimental, Eye, American Journal of Cardiology, and others.
The Department of Molecular Genetics, led by Dr. Radha Venkatesan, was established in December 1999 with the mission to unravel the genetic basis of diabetes, its complications, cardiovascular diseases, and obesity. The department focuses on molecular and functional genomics research, supported by prestigious funding agencies such as ICMR, DBT, and DST. Over the years, it has made remarkable contributions to the field through high-impact publications in leading international journals, including Nature, Nature Genetics, Nature Communications, American Journal of Human Genetics, Cell Genomics, Clinical Genetics, Metabolism, Diabetologia, and Diabetes Care. The department continues to advance the understanding of genetic mechanisms underlying metabolic disorders, paving the way for personalized medicine and targeted therapies.

Dr. Radha Venkatesan
Department of Molecular Genetics
In India, our department is known for development of basic science discoveries into clinical applications, that is, “bench to bedside” work. This has been possible through our genomics work on monogenic diabetes and CHI subtypes where we investigate their genetic and molecular aetiology.
Monogenic diabetes encompasses a heterogeneous group of single-gene disorders that affect pancreatic β-cell function, leading to impaired insulin secretion.
The primary categories of monogenic diabetes include:
- Maturity Onset Diabetes of the Young (MODY)
- Neonatal Diabetes Mellitus (NDM)
- Syndromic forms of diabetes, which involve additional systemic features
All these forms manifest as hyperglycemia due to insufficient insulin secretion.
In contrast, Congenital Hyperinsulinism (CHI) represents a monogenic disorder characterized by excessive insulin secretion from pancreatic β-cells, leading to persistent hypoglycemia, due to single gene mutations.
Maturity Onset Diabetes of the Young (MODY) is a rare, inherited form of diabetes that typically appears in adolescence or early adulthood, but unlike type 1 or type 2 diabetes, it is caused by a single gene mutation and follows an autosomal dominant inheritance pattern.
We are actively addressing Congenital Hyperinsulinism (CHI), a genetically heterogeneous disorder characterized by persistent hypoglycemia. If untreated, CHI poses a serious risk to neurodevelopment. By identifying the underlying genetic causes, we offer targeted treatment strategies, significantly improving clinical outcomes in these children.
Given the high prevalence of consanguinity in certain Indian communities, coupled with India’s large and genetically diverse population, our research holds significant public health relevance. Accurate diagnosis and genetic characterization are essential for effective management and prevention strategies.
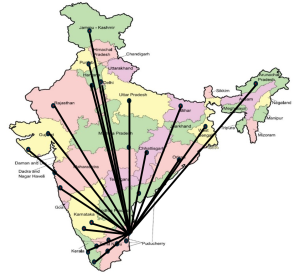
We have established a registry for monogenic diabetes and CHI patients currently totalling 2319 , referred by Paediatricians and Neonatologists from across the country.
Our work is ongoing and longitudinal in nature. We continue to monitor patient outcomes over time to better understand disease progression and optimize treatment protocols. This research serves as a model for implementing precision medicine in complex and resource-limited settings.
Neonatal Diabetes Mellitus (NDM), which manifests within the first year of life, was historically misdiagnosed as Type 1 Diabetes, leading to lifelong insulin therapy with suboptimal outcomes. Our research, along with global efforts, has demonstrated that a significant proportion of NDM cases are due to specific genetic mutations. Importantly, many of these patients respond remarkably well to oral sulfonylurea therapy, allowing for successful transition from insulin injections to tablets. This shift has dramatically improved quality of life and long-term prognosis.
We have played a pioneering role in demonstrating the clinical actionability of specific genetic mutations in NDM—translating bench side discoveries directly to bedside care. A crucial part of our work involves differentiating between pathogenic mutations and benign variants through robust experimental validation. We are the only laboratory in India equipped to functionally assess these variants for their disease-causing potential and clinical relevance and actionability. This approach exemplifies precision medicine, providing tailored treatment strategies that are both effective and sustainable.
The lack of knowledge of the functional consequences of the variants, lead to false assignments of causality at the variant level rendering their pathogenicity status ambiguous, thus resulting in incorrect treatment for patients harbouring these variants. Therefore, a thorough knowledge of functional implications of variants becomes imperative.We have performed functional characterization of the gene variants, moving from variant level classification based on functional characterization to understanding the pathogenicity and potential clinical actionability of these variants.We have also successfully compiled “lookup” tables and reinterpretations for many genetic variants in HNF1A, ABCC8, KCNJ11, HNF4A, and NKX2.2 genes.
First in the world discovery
Very recently, we have discovered a novel subtype of maturity-onset diabetes of the young (MODY) category, known as ABCC8-MODY. Traditionally, ABCC8 mutations are known to act through gain-of-function (GOF) mechanisms, leading to increased activity of the ABCC8 protein. GOF mutations are classically associated with neonatal diabetes when they occur in infancy, and with ABCC8-MODY (also known as MODY 12) in adults.
In contrast, our laboratory investigations have identified novel ABCC8 mutations in Indian patients with MODY that act through a loss-of-function (LOF) mechanism. LOF mutations reduce or abolish the activity of the ABCC8 protein, impairing the function of KATP channels in the β-cell membrane. These types of mutations are typically associated with congenital hyperinsulinism (CHI), a condition characterized by persistent hypoglycemia in childhood. Interestingly, patients in our cohort appeared to exhibit CHI in early life, which then crossed over to diabetes later in adulthood. Based on our genetic and functional studies, we proposed that diabetes caused by KATP channel mutations should be classified as distinct disease subtypes, namely GOF ABCC8 MODY and LOFABCC8 MODY.
An important clinical implication of our discovery is that patients with this new LOF subtype of ABCC8-MODY do not respond to sulfonylureas, which are effective in other MODY forms such as MODY 1, MODY 3, and even traditional ABCC8-MODY (MODY 12). This necessitates further research into alternative antidiabetic treatments for this group. Our findings emphasize the critical role of genetic testing followed by functional assays in enabling precision diagnosis and personalized care for individuals with monogenic diabetes. Furthermore, this study opens new avenues for the identification of novel drug targets and the development of tailored therapies in diabetes management.
We were recognized as ICMR nodal centre for monogenic diabetes in India, through which we have performed genetic screening for more than 2300 children affected with such a condition , all over the country
Genetics of polygenic type 2 diabetes(T2D)
We were one of the early research groups in the country to work on the association of genetic variants ( SNPs)with type 2 diabetes and related traits. We started out by testing genes one by one but later expanded this to replication and GWAS stidies. We were part of the group where we identified six novel loci associated with type 2 diabetes which made its way toa publication in the prestigious journal Nature Genetics. Recently,we have performed a large scale (27000 T2D patients) Genome Wide Association study (GWAS) which gives genotypic profiles of the patients and groups individuals into different clusters of genotypes and phenotypes.
Previously we were a part of the Genome Asia100 K consortium which sequenced genomes of 1267 individuals from diverse groups from the country. We are now poised to undertake a large-scale whole genome sequencing (WGS) initiative focused on type 2 diabetes at the Madras Diabetes Research Foundation (MDRF).This is a collaboration with Emory University in the US and AIIMS and CCDC, IN Delhi , India. This project will encompass approximately 20,000 individuals and represents one of the largest WGS efforts in the Indian population to date. The data generated is expected to reveal critical insights into the genetic architecture of type 2 diabetes and highlight the unique genomic signatures of the Indian population.
Genetics of Diabetic Kidney Disease (DKD)
Candidate gene association studies conducted to understand the genetic basis of diabetic kidney disease (DKD) linked DKD with polymorphisms in CNDP1 and TCF7L2. GWAS-identified variants in SLC12A3 and ELMO1 which were replicated in the South Indian population showed significant association with DKD. A genome-wide association study (GWAS) for DKD has been conducted in the Indian T2D population to identify risk variants and develop a polygenic risk score for prediction. This is the first GWAS of DKD in the Indian population, and data analysis is currently underway. In addition to genetic variants, DNA methylation can influence susceptibility to DKD by altering gene expression. To explore this, an epigenome-wide association study (EWAS) is being conducted using the Infinium MethylationEPIC array (935,000 CpG sites). This study aims to evaluate the combined predictive value of genetic and epigenetic markers for DKD in Indians with T2D.
Genetic etiology of congenital renal abnormalities in Indian diabetic patients using exome sequencing
Congenital anomalies of the kidney and urinary tract (CAKUT) are a leading cause of end-stage renal disease (ESRD) and are frequently linked to early-onset diabetes, including MODY5. This study aims to perform whole-exome sequencing (WES) in young Indian patients with diabetes and congenital renal abnormalities to identify and validate pathogenic variants. The study may reveal new genetic factors underlying renal abnormalities in diabetic patients.

Future research plans
- To continue to identify the Identification of genetic etiology of MODY, Neonatal diabetes, syndromes of neonatal diabetes and Congenital Hyperinsulinism towards translational genomic research and precision medicine in India. The finding that genetic etiology strongly influences treatment choices and the clinical course of the disease makes it a high priority research, thereby have a tremendous translational potential from ‘bench to bedside’.
- Molecular characterization of identified variants using experimental pipeline in order to establish functional explanation for naturally occurring variants in Monogenic diabetes and CHI patients. Combining functional follow up and segregation studies in order to assess the clinical actionability of variants towards precision medicine. This study is expected to help in the understanding the underlying molecular etiology and treatment plan.
- To continue to create “look up” tables for instant reference in clinical settings for treating monogenic diabetes and CHI
- To construct a polygenic risk score for gestational diabetes, type 1 and type 2 diabetes patients in Indian population.
- To develop the biological resource to advance the diagnosis and understanding of NDM and CHI in India using iPSC model system.

Facilities
The Molecular Genetics Department at MDRF is equipped with cutting-edge technologies dedicated to advancing genetic research. The laboratory is specialized in genotyping and sequencing technology, quality control procedures and is developing throughput procedures for most of the genetic investigations in addition to developing functional genomics studies. The major instrumentation includes Illumina iScan microarray and Realtime PCR (ABI, Quad Studio5); Golden standard sequencing facility -Genetic Analyzer, ABI3500; Spectramax i3X Microplate Luminometer – luminescence detection system for expression studies including reporter assays and Electroporator for express delivery of nucleic acids, vector constructs into cells. In addition to other smaller equipments such as GeneAmp-PCR Systems (Dual 384 block), PCR machines (96 well), BioRad Gel documentation system, Electrophoresis apparatus, Centrifuges, etc.
The department is set to further enhance its capabilities with the installation of the Illumina NovaSeq X Plus – a state-of-the-art next-generation sequencing (NGS) platform shortly. This will significantly expand the lab’s capacity to conduct whole genome sequencing (WGS), whole exome sequencing (WES), targeted sequencing (tNGS), amplicon sequencing and transcriptomics (RNA Seq).
The department is transforming medicine by expanding the role of genetics and genomics in science and medicine through discoveries and integration of basic and clinical research.
MONOGENIC DIABETES REGISTRY
We at Madras Diabetes Research Foundation and Dr. Mohan’s Diabetes Specialities Centre have launched the monogenic diabetes registry, the first nationwide database of individuals affected by the rare forms of diabetes. The purpose of the registry is to track and study the monogenic forms of diabetes on a long term basis, to understand the genetic patterns in the families and to deliver optimal therapies for each patient according to their genetic etiologies. As a part of this, we perform genetic testing for patients affected with neonatal diabetes or MODY subtypes of diabetes and for their families.

Interested in collaborations ?