The Department of Vascular Biology at the Madras Diabetes Research Foundation (MDRF) is dedicated to advancing innovative research that promotes and optimizes vascular health, particularly in individuals living with diabetes. Established in 2011, the department focuses on understanding both microvascular and macrovascular complications associated with diabetes—conditions that significantly impact patient quality of life. By investigating early molecular changes and identifying novel biomarkers under varying glucose levels, the department aims to enhance early diagnosis, prevention, and targeted therapeutic strategies for vascular diseases in diabetes. In addition to research, the department contributes to capacity-building by offering hands-on training to students and researchers from external institutions in advanced cell culture and molecular biology techniques.
The Department of Vascular Biology is led by Dr. Nagaraj Manickam, Ph.D, a distinguished scientist with over twenty years of experience in the field of diabetic vascular complications. His extensive expertise includes six years of post-doctoral research in the United States, where he gained advanced exposure to cutting-edge vascular research methodologies. Under his leadership, the department has expanded its scientific focus, integrating molecular signaling studies, biomarker discovery, and translational approaches to better understand vascular dysfunction in diabetes. Dr. Manickam’s vision and commitment continue to guide the department toward impactful scientific contributions that improve vascular care and patient outcomes.

Dr. Nagaraj Manickam
Research Activities
Role of Gasotransmitters and Treatment Strategies to combat DKD
In our previous study, we found that nitric oxide (a Gasotransmitter) was decreased upon ADMA accumulation. Recent studies are also pointing towards the role of other Gasotransmitters on the vasculature. Hence, we have extended our aim to study the role of Gasotransmitters in diabetic kidney disease and the regulation of Gasotransmitter metabolism. Further, we have selected an Indian herbal plant (Aegle marmelos – AM) to study the protective role of its leaf extract and its active phytochemical components on diabetic kidney disease. We elucidated the possible molecular and cellular mechanism of AM in retarding the progression of nephropathy using diabetes induced C57BL/6 mice and in vitro models.
MicroRNAs/Sestrin2/Atherosclerosis/Diabetes Relationship
In our previous study we have identified and proved that Sestrin2 counteracts monocyte activation under hyperglycemic and dyslipidemic conditions. Further, our studies point out that there exists impaired sestrin2 and its downstream signaling in diabetes and its cardiovascular consequences. But the reason for the decrease in the sestrin2 expression under stress condition such as hyperglycemic/hyperlipidemic states is not clearly understood. Recent studies imply a role of miRNAs in altering gene expression and biological activities. Therefore, targeting miRNAs has recently been conceived as a potential strategy to beneficially modulate the biomarkers
Cardiovascular Disease (CVD) / Coronary Artery Disease (CAD)
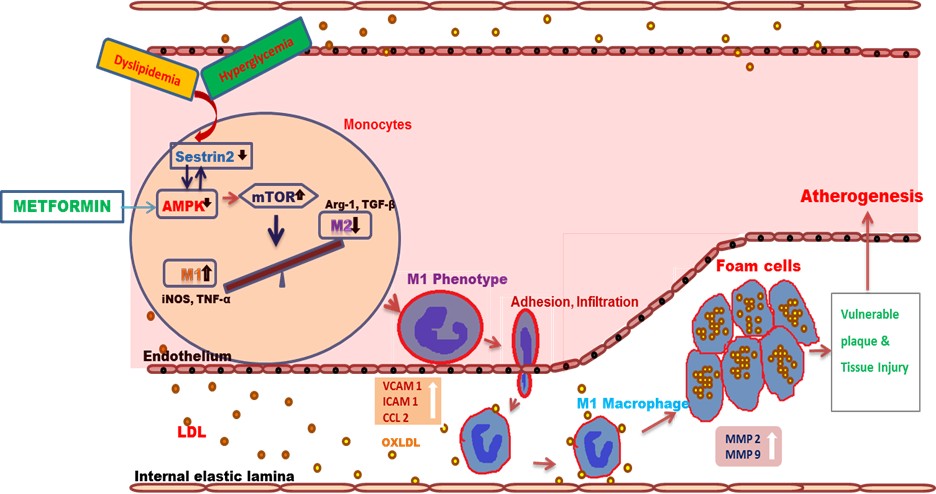
Our other aim is to look at the macro vascular complications of diabetes. Atherosclerosis is a leading cause of Cardiovascular disease in the metabolic syndrome. Hence, our focus is to find a novel biomarker for the disease and to find a drug target to impede the progression of the disease. After a great deal of experiments using different molecular biology techniques, we have found that a stress inducible protein called Sestrin2 has been significantly decreased on high glucose or OxLDL treatments in the monocytes and that the decrease in Sestrin2 leads to monocyte activation, trans-differentiation and foam cell formation (key events in the process of atherosclerosis) through the regulation of AMPK and mTOR. It is the first of its kind in dissecting out a molecular signaling pathway involving sestrin2-mTOR under high glucose and dyslipidemic conditions
Further, we have found the status of Sestrin2 in the monocytes and circulation of people with/without diabetes and/or dyslipidemia. We have found a significant association and correlation of sestrin2 levels with lipid parameters.
Further to understand the role of Sestrin2, we have utilized animal models and found that the sestrin2 expression levels were decreased in the aorta and heart tissues of Wistar rats.
Training Programs at our Department
Our department offers comprehensive training and internship programs for graduate and post-graduate students in life-sciences. Students from across the nation regularly join our department to carry out their final-year project work, gaining hands-on research experience in a dynamic academic environment.
Facilities and Technologies
Our department is fully equipped with state-of-art facilities to support in vitro molecular signaling studies including a dedicated cell culture laboratory, confocal microscopy, RT-qPCR, gel documentation system, multimode reader and more.
Awards and Recognition
Our original research article published in the ‘Journal of Lipid and atherosclerosis’ was honored as the Best Original Paper of the year (2024) at International Congress on Lipid and Atherosclerosis, Seoul, Korea, 2024
Publications
- Venkatesan S, Rajagopal A, Muthuswamy B, Mohan V, Manickam N. Phytochemical Analysis and Evaluation of Antioxidant, Antidiabetic, and Anti-inflammatory Properties of Aegle marmelos and Its Validation in an In-Vitro Cell Model. Cureus. 2024 Sep 30;16(9):e70491. doi: 10.7759/cureus.70491. PMID: 39479139; PMCID: PMC11523027.
- Rajagopal, A; Venkatesan, S; Balasubramanyam, M; Anjana, R. M; Mohan, V; Manickam, N. Targeting miR-122 and miR-4756 mitigates atherogenic events by upregulating Sestrin2 under high glucose condition. Diabetes Research and Clinical Practice. 2024; 209: 111218
- Sundararajan S, Jayachandran I, Pandey GK, Venkatesan S, Rajagopal A, Gokulakrishnan K, Balasubramanyam M, Mohan V, Manickam N. Metformin Reduces the Progression of Atherogenesis by Regulating the Sestrin2-mTOR Pathway in Obese and Diabetic Rats. J Lipid Atheroscler. 2023 Sep;12(3):290-306.
- Jayachandran, I; Sundararajan, S; Venkatesan, S; Rajagopal, A; Anjana, RM; Mohan, V; Manickam, N. IDF21-0159: Asymmetric Dimethylarginine and 20-Hydroxyeicosatetraenoic Acid–A lethal combo in diabetic kidney disease. Diabetes Research and Clinical Practice. 2022; 186: 7.

Interested in collaborations ?